
#GAMP 5 SOFTWARE CATEGORIES HOW TO#
Do you want to know which cloud solutions to choose and how to qualify cloud-based infrastructure? We invite you for training.ĭata integrity for computerized systems and validation of mobile applications Many companies move their IT resources to the cloud and GxP compliant qualification can bring many questions. Increasingly, however, the infrastructure is in the cloud, which causes major modifications in the current activities of IT departments and quality departments dealing with the validation of computerized systems. Systems in the GxP environment must be validated and infrastructure qualified. It also shows how to design spreadsheets and what design techniques to use for each sheet category.Ĭloud Validation - Qualification of cloud-based IT infrastructure according to GAMP The training includes a practical approach to validation issues of spreadsheets made in MS Excel 365 according to GAMP 5. Validation of MS Excel spreadsheets according to GAMP 5 and good practices in the pharmaceutical industry
#GAMP 5 SOFTWARE CATEGORIES SOFTWARE#
This training is dedicated to both software suppliers for GxP regulated companies, especially for the pharmaceutical industry and medical solutions, including software as a medical device as well as for System Owners and QA (quality control) dealing with the validation of such solutions in pharmaceutical companies and regulated GxP. How to connect the world of so-called development with the world of validation? There is a proven way to do it. The Life Science GxP requirements for validation are based on GAMP 5 and the waterfall and V-Model approach. Software and systems development is usually carried out by Scrum-based programming teams. Do you want to learn how to validate computerized systems from the consulting side? We invite you to a two-day dedicated training with practical examples and workshops.Īgile GxP validation - Validation using the Scrum methodology
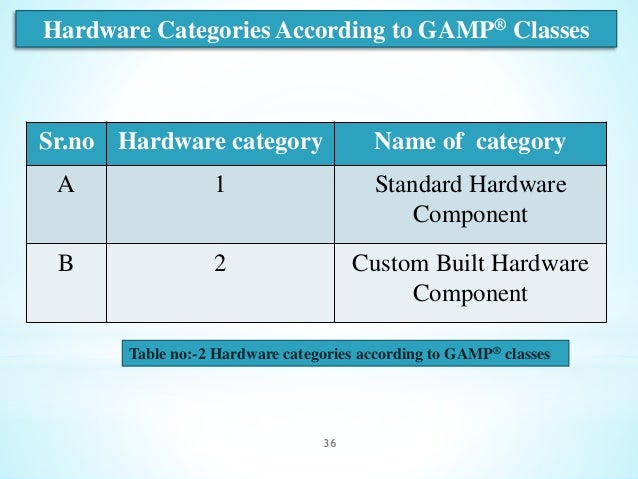
In addition, it is interpreted differently by auditing institutions and various pharmaceutical companies using their developed practices. However, the guide, although concise, is only a general guide and does not fully cover issues related to the validation of computerized systems. GAMP 5 is a well-known guide that helps a company subject to GxP to validate and maintain computerized systems in a validated state. The training is based on the recommendations of GAMP 5. The training includes a practical approach to the issues of validation and maintenance of computerized systems of different categories. Validation and maintenance of computerized systems Validation of computerized systems - extension for practitioners.Infrastructure qualification - a qualification including virtual networks and IaaS, PaaS, SaaS models compared to the on-premise model.Excel validation - excel sheet validation, sheet categorization.Agile validation - Validation using the Agile Scrum methodology for GxP.Validation and maintenance of computerized systems according to GAMP 5, 2-day training.Training can be conducted in Polish and English:

We run the following training in the area of computerized systems validation. Computer Systems Validation Academy - training in the broadly understood validation of computer systems


 0 kommentar(er)
0 kommentar(er)
